 They say that life is one continuous process of aging, which inevitably ends in death. This may not sound very optimistic, but such are the laws of nature. Aging and death are some of the most important regulators of a population of any kind on the planet, and immortality in the biological sense of the word exists only in books and movies. The same situation is with "eternal" youth. In the books we have Dorian Gray - an eternally young and handsome young man with a strong antipathy to a particular work of art; Edward Cullen is an eternally young vampire with vitamin D deficiency and high skin reflectivity, etc. In reality, eternal or at least prolonged youth is unattainable. At least if scientists do not interfere in the process. Today we will get to know the study,in which scientists from Stanford University were able to achieve rejuvenation of old human cells due to certain proteins involved in the process of embryonic development. What substances were used, how old the cells were younger, and how long was the effect? We learn about this from the report of the research group. Go.
They say that life is one continuous process of aging, which inevitably ends in death. This may not sound very optimistic, but such are the laws of nature. Aging and death are some of the most important regulators of a population of any kind on the planet, and immortality in the biological sense of the word exists only in books and movies. The same situation is with "eternal" youth. In the books we have Dorian Gray - an eternally young and handsome young man with a strong antipathy to a particular work of art; Edward Cullen is an eternally young vampire with vitamin D deficiency and high skin reflectivity, etc. In reality, eternal or at least prolonged youth is unattainable. At least if scientists do not interfere in the process. Today we will get to know the study,in which scientists from Stanford University were able to achieve rejuvenation of old human cells due to certain proteins involved in the process of embryonic development. What substances were used, how old the cells were younger, and how long was the effect? We learn about this from the report of the research group. Go.Study basis
What is aging? At its core, this is a process of gradual loss of functionality at the level of molecules, cells, tissues and the whole organism.At the chromatin * level, aging is associated with a progressive accumulation of epigenetic * errors, which ultimately lead to aberrant (abnormal) gene regulation, stem cell depletion, aging and impaired cell / tissue homeostasis.Chromatin * - the basis of chromosomes, consisting of DNA and proteins (mostly histones). Chromatin is located inside the nucleus of eukaryotic cells (organisms that have a nucleus in the cells) and is part of the nucleoid in prokaryotes (unicellular without a nucleus).
Epigenetic inheritance * - a set of inherited changes in the phenotype or gene expression.
If you apply nuclear reprogramming of pluripotency * cells, you can reverse it as its age, but not identity.Pluripotency * is a characteristic of a cell that can differentiate into all types of cells, except cells of extra-germ organs.
Previously, this technique has already been tested on mice. The results were very encouraging, as temporary reprogramming improved age characteristics and prolonged life span in mice. But man is not a mouse, and therefore the question remains how this technique will work on human cells. This is exactly what scientists are considering in this study.The process of nuclear reprogramming into induced pluripotent stem cells ( iPSCs * ) is characterized by a complete reset (reset) of the epigenetic characteristics of cells, which leads to the return of both cellular identity and age to an embryo-like state.Induced pluripotent stem cells (iPSCs) * are a type of stem cells obtained in the laboratory from mature differentiated cells of a mammalian organism (including humans).
It is curious that reprogramming is not irreversible, stopping to the so-called point of no return (PNR from point of no return), after which the cells eventually return to their original somatic * state.Somatic cell * - cells that make up the soma (body of the body), but not involved in sexual development.
Therefore, if reprogramming will affect the cell for a fairly short period of time, then the expression of reprogramming factors will not be able to erase the epigenetic signature that determines the identity of the cells. It turns out that a short exposure will not change the identity of the cell, but can it affect its age?The first evidence that temporary reprogramming can improve phenotypes during aging was shown in mice. But, as scientists have already reminded us, mice are not humans. An important question of this technique is whether it can work on naturally occurring human cells isolated from older people.To find out, scientists conducted a series of experiments to identify the degree of impact of nuclear reprogramming on the phenotypes (external and internal signs) of human and mouse cells.Research results
First, an assessment was made of the effect of transient expression of reprogramming factors on the transcript of two different types of cells ( fibroblasts * and endothelial * cells) in the elderly. Next, the transcript of the elderly was compared with the transcript of the same cell types of young donors ( 1a and 1e ).Fibroblast * - cells of the connective tissue of the body that synthesize the extracellular matrix.
Endothelium * - a layer of flat cells on the inner walls of blood vessels, lymphatic vessels and cardiac cavities.
 Image No. 1Fibroblasts were obtained from biopsy samples * of the skin of the hands and abdomen of the study participants: 3 people aged 25–35 years (young group) and 8 people aged 60–90 years (elderly group).
Image No. 1Fibroblasts were obtained from biopsy samples * of the skin of the hands and abdomen of the study participants: 3 people aged 25–35 years (young group) and 8 people aged 60–90 years (elderly group).Biopsy * - biological material obtained by biopsy.
Endothelial cells were extracted from the iliac vein and artery of the study participants: 3 people aged 15–25 years (young group) and 8 people aged 50–65 years (elderly group).For the experiments, a non-integrative reprogramming protocol was used, which was optimized based on a set of mRNA expressing OCT4, SOX2, KLF4, c-MYC, LIN28, and NANOG proteins.This protocol sequentially produces iPSC colonies, regardless of the age of the donors, after 12–15 daily transfections * .Transfection * is the process of introducing nucleic acid into eukaryotic cells by the non-viral method.
It was found that the point of no return in this case is observed on the fifth day of reprogramming. This conclusion is based on the fact that the first detectable expression of endogenous pluripotency- associated dcRNA * occurs on day 58.dnaRNAs (long non-coding RNAs) * are RNA molecules located in the nucleus or in the cytoplasm that are not translated into proteins.
In view of this, it was decided to use a temporary regime of exogenous expression in which OSKMLN was transfected daily for 4 consecutive days, and gene expression analysis was performed 2 days after interruption ( 1b ).Next, paired mass RNA sequencing was performed for both types of cells of all three groups of subjects: young (Y), elderly without reprogramming (UA) and elderly with reprogramming (TA).To begin with, scientists compared the quantile normalized transcripts of young subjects and the elderly without reprogramming (Y and UA). The analysis showed that 961 genes (5.85%) in fibroblasts ( 1a and 1c ) and 748 genes (4.80%) in endothelial cells ( 1e and 1f) differed between young and old cells.These gene sets are directly related to aging processes. Determining the direction of expression above or below the average value of each gene showed a clear similarity between the cells of young people and the cells of the elderly after reprogramming for both fibroblasts and endothelial cells ( 1d and 1g ).Using the method described above, scientists compared cell populations of elderly subjects with and without reprogramming (TA and UA; 1a and 1e ). It was found that 1042 genes in fibroblasts and 992 in endothelial cells were differentially expressed.These transcriptome profiles were then used to confirm preservation of cell identity after reprogramming. The analysis showed that there were no significant changes in identity.In general, analysis of transcriptome signatures showed that OSKLMN expression promotes very rapid activation of a younger gene expression profile without affecting cell identity gene expression.Researchers note that epigenetic clocks * based on DNA methylation levels * are the most accurate molecular biomarkers of age in tissues and cells. They are also predictive of many age-related conditions, including life expectancy.* — , , .
* — .
It is also known that exogenous expression of canonical reprogramming factors (OSKM) returns the epigenetic age of primary cells to the prenatal state.To test whether transient expression of OSKMLN can reverse the epigenetic clocks of human somatic cells, scientists used two types of epigenetic clocks that apply to fibroblasts and human endothelial cells: the original Panvis Horvath epigenetic clock (based on 353 pairs of cytosine-phosphate-guanine) and later “skin-blood” watches (based on 391 CpGs - DNA molecule sites).Howarth Epigenetic Age CalculatorAccording to Howart's epigenetic clock, the temporary exposure to OSKMLN significantly reversed the age of DNA methylation (mean age difference = -3.40 years). The effect of rejuvenation was more pronounced in endothelial cells (average age difference = -4.94 years; 1i ) than in fibroblasts (average age difference = -1.84; 1h ).Qualitatively similar, but less significant results were obtained using a skin-blood clock: the overall effect of rejuvenation is -1.35 years; the average rejuvenation in endothelial cells is -1.62 and in fibroblasts -1.07.Based on these results, an analysis was made of the effect of temporary reprogramming on various signs of cellular physiological aging. For this, visualization of individual cells and an extensive database of signs of aging were carried out.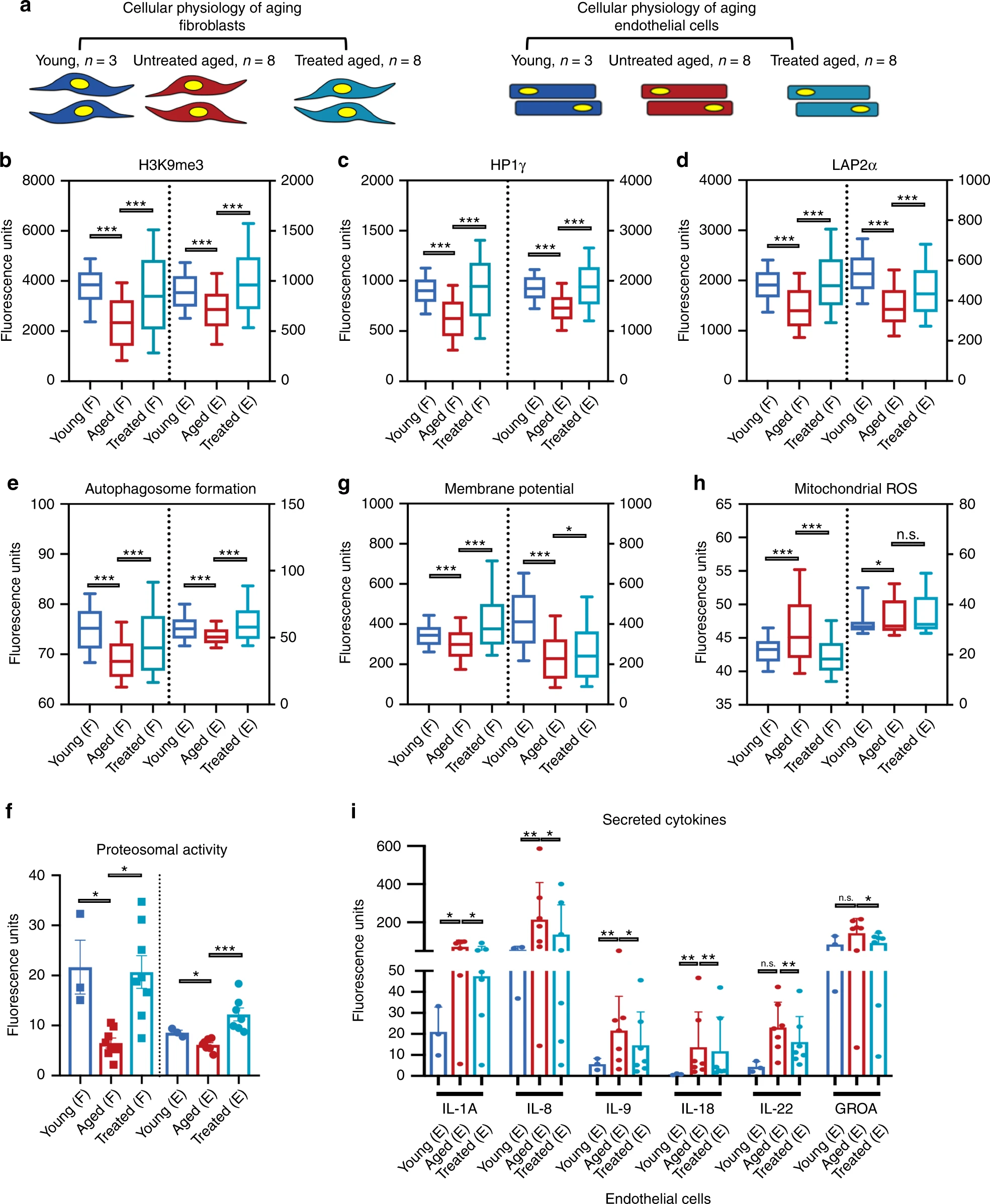 Image No. 2All analyzes were carried out separately in each separate cell line ( 2a ): 19 lines of fibroblasts (3 - young, 8 - elderly and 8 - elderly after reprogramming) and 17 lines of endothelial cells (3 - young, 7 - elderly and 7 - elderly after reprogramming). Statistical analysis was carried out by random sampling of 100 cells per sample.To expand on the previous results on epigenetics, additional quantitative measurements were carried out using immunofluorescence (IF) of the epigenetic repressive label H3K9me3, heterochromatin-associated protein HP1γ and protein LAP2α supporting the nuclear laminin * ( 2b - 2d ).
Image No. 2All analyzes were carried out separately in each separate cell line ( 2a ): 19 lines of fibroblasts (3 - young, 8 - elderly and 8 - elderly after reprogramming) and 17 lines of endothelial cells (3 - young, 7 - elderly and 7 - elderly after reprogramming). Statistical analysis was carried out by random sampling of 100 cells per sample.To expand on the previous results on epigenetics, additional quantitative measurements were carried out using immunofluorescence (IF) of the epigenetic repressive label H3K9me3, heterochromatin-associated protein HP1γ and protein LAP2α supporting the nuclear laminin * ( 2b - 2d ).Nuclear Lamin * - a fibrillar rigid network under the nuclear membrane, which is involved in the organization of chromatin.
As previously reported in previous studies, in mature fibroblasts and endothelial cells, a decrease in nuclear signal was observed for all three markers compared to young cells. Reprogramming of older cells has led to an increase in these markers in both types of cells.Next, the proteolytic (protein hydrolysis) activity of cells was studied by measuring the formation of autophagosomes, and the chemotrypsin-like proteasome activity was also studied. Reprogramming not only increased both types of activity to the level of young cells, but also exceeded it ( 2e and 2f ). This suggests that the early stages of reprogramming contribute to the active clearance of degraded biomolecules.One of the most indicative signs of cell aging is a decrease in mitochondrial activity, the accumulation of reactive oxygen species (ROS), and dysregulation of nutrient sensitivity. Therefore, it is possible to check the effect of reprogramming on aging cells by measuring the membrane potential of mitochondria, ROS mitochondria and protein levels of Sirtuin1 (SIRT1).Temporary reprogramming increased the membrane potential of mitochondria in both cell types ( 2g ), decreased mitochondrial ROS ( 2h ), and increased SIRT1 protein levels in fibroblasts, which is observed in young cells.Cell imaging by staining beta-galactosidase associated with aging showed a significant decrease in the number of aging cells in endothelial cells, but not in fibroblasts. This was accompanied by a decrease in the amount of secretory phenotypic cytokines associated with pro-inflammatory aging, and again in endothelial cells, but not in fibroblasts ( 2i ).It was also found that in none of the cell types does the telomere * length show significant elongation after reprogramming. This may indicate that the cells do not differentiate into a state similar to stem cells when telomerase * activity is reactivated.* — .
* — , 3'- .
It was also found that the effects of reprogramming in cells persisted even after 4 and 6 days after this process stopped. From the point of view of the speed of manifestation of effects from reprogramming, they were observed already 2 days after the start of this process.In total, these data suggest that the transient expression of OSKMLN can cause rapid, continuous improvement and change in cell age in human somatic cells at the transcriptome, epigenetic, and cellular levels. In addition, this rejuvenation occurs in the early stages of reprogramming, due to which this technique does not have time to affect the identity of the cells.The scientists then decided to find out if transient expression of OSKMNL could also reverse the inflammatory phenotypes associated with aging. After obtaining preliminary evidence of this change in endothelial cells ( 2j ), the analysis was expanded by adding osteoarthritis, a disease strongly associated with aging and characterized by a pronounced inflammatory process affecting chondrocytes in the joints.Samples were taken - chondrocytes from the cartilage of six patients aged 60–70 years who underwent a complete joint replacement operation due to late stage osteoarthritis. Further, these samples were reprogrammed and the results of this were compared with the data of chondrocytes of three young people ( 3a ). Image No. 3Temporary expression of OSKMLN was performed for 2 or 3 days, and the analysis was performed 2 days after interruption of reprogramming, although the patients had a more lasting effect with longer treatment.However, the use of OSKMLN showed a significant decrease in intracellular mRNA levels of RANKL (cytokine of the family of tumor necrosis factors) and iNOS2, as well as levels of inflammatory factors secreted by cells ( 3b - 3d ).In addition, an increase in cell proliferation ( 3e ), an increase in ATP production ( 3f ), and a decrease in the oxidative process ( 3g and 3h ) were observed .Verification of cell identity showed that the reprogramming process did not violate it: there was no effect on the level of SOX9 expression, and the level of COL2A1 expression increased significantly (qRT-PCR on 3i and 3j ).From the above results it follows that the transient expression of OSKMLN can contribute to a partial reversal of gene expression and cell physiology in older chondrocytes with osteoarthritis to a younger state. Therefore, the studied technique can also be used as one of the methods of treating osteoarthritis in the elderly.Another important sign of aging is the loss of stem cell function and the loss of regenerative ability. To test the effect of reprogramming on these factors, scientists used mouse skeletal muscle stem cells (MuSC).MuSCs were exposed to reprogramming for 2 days while they were at rest. Individuals aged 3 months and 20-24 months ( 4a ) served as sample donors .
Image No. 3Temporary expression of OSKMLN was performed for 2 or 3 days, and the analysis was performed 2 days after interruption of reprogramming, although the patients had a more lasting effect with longer treatment.However, the use of OSKMLN showed a significant decrease in intracellular mRNA levels of RANKL (cytokine of the family of tumor necrosis factors) and iNOS2, as well as levels of inflammatory factors secreted by cells ( 3b - 3d ).In addition, an increase in cell proliferation ( 3e ), an increase in ATP production ( 3f ), and a decrease in the oxidative process ( 3g and 3h ) were observed .Verification of cell identity showed that the reprogramming process did not violate it: there was no effect on the level of SOX9 expression, and the level of COL2A1 expression increased significantly (qRT-PCR on 3i and 3j ).From the above results it follows that the transient expression of OSKMLN can contribute to a partial reversal of gene expression and cell physiology in older chondrocytes with osteoarthritis to a younger state. Therefore, the studied technique can also be used as one of the methods of treating osteoarthritis in the elderly.Another important sign of aging is the loss of stem cell function and the loss of regenerative ability. To test the effect of reprogramming on these factors, scientists used mouse skeletal muscle stem cells (MuSC).MuSCs were exposed to reprogramming for 2 days while they were at rest. Individuals aged 3 months and 20-24 months ( 4a ) served as sample donors .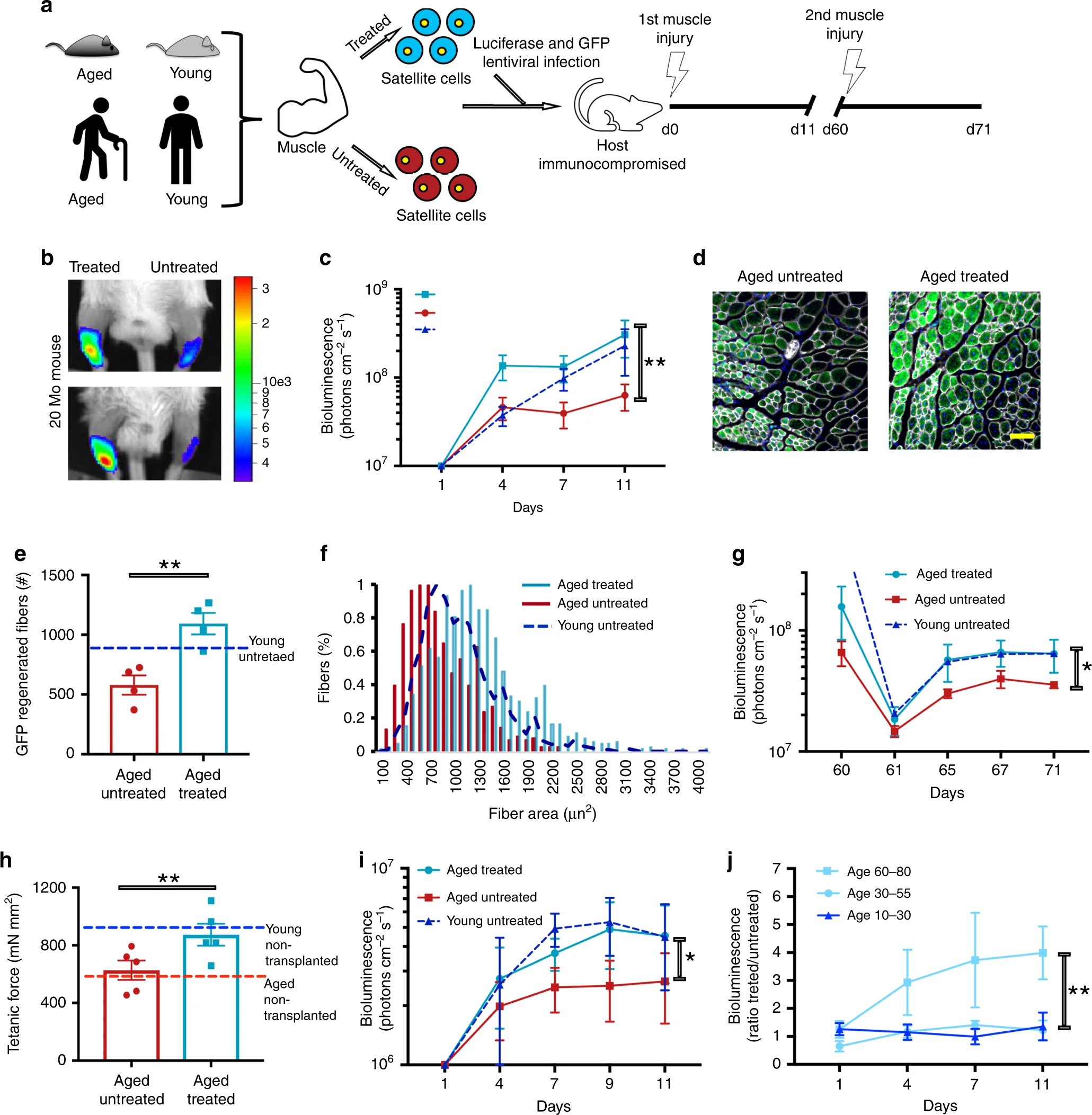 Image No. 4Processing of old MuSC decreased both the time of the first division, approaching it to the faster kinetics of activation of young MuSC cells, and the mitochondrial mass. Moreover, reprogramming partially restored the reduced ability of individual MuSCs to form colonies.Next, a test was carried out of the functionality and effectiveness of MuSC regarding the regeneration of new tissue. For this, transduction of young, elderly, or temporarily reprogrammed MuSC cells was performed using lentivirus-expressing luciferase and GFP, which were then transplanted into damaged muscles of the anterior tibia of immunocompromised mice.Bioluminescent imaging initially showed that the muscles in which the reprogrammed MuSCs were placed showed the highest signal (day 4; 4b and 4c ). However, on day 11, these muscles became similar to the muscles where young MuSCs were added. But the muscles where the aging MuSC cells were added without reprogramming showed the weakest signals ( 4b and 4c)Immunofluorescence analysis showed that cells with reprogramming provided tissues with more myofibrils than cells without treatment ( 4d and 4e ). 60 days after the first analysis, another was performed that showed the same results ( 4g ). This directly indicates an improvement in the regenerative ability of tissues after exposure to cells that have undergone reprogramming.At the next stage of the study, special attention was paid to age-related atrophic degenerative muscle changes. Scientists decided to check whether their technique can restore the muscle strength of elderly mice to the level of young.First, tetanic * strength was established in the muscles of mice aged 4 and 27 months.
Image No. 4Processing of old MuSC decreased both the time of the first division, approaching it to the faster kinetics of activation of young MuSC cells, and the mitochondrial mass. Moreover, reprogramming partially restored the reduced ability of individual MuSCs to form colonies.Next, a test was carried out of the functionality and effectiveness of MuSC regarding the regeneration of new tissue. For this, transduction of young, elderly, or temporarily reprogrammed MuSC cells was performed using lentivirus-expressing luciferase and GFP, which were then transplanted into damaged muscles of the anterior tibia of immunocompromised mice.Bioluminescent imaging initially showed that the muscles in which the reprogrammed MuSCs were placed showed the highest signal (day 4; 4b and 4c ). However, on day 11, these muscles became similar to the muscles where young MuSCs were added. But the muscles where the aging MuSC cells were added without reprogramming showed the weakest signals ( 4b and 4c)Immunofluorescence analysis showed that cells with reprogramming provided tissues with more myofibrils than cells without treatment ( 4d and 4e ). 60 days after the first analysis, another was performed that showed the same results ( 4g ). This directly indicates an improvement in the regenerative ability of tissues after exposure to cells that have undergone reprogramming.At the next stage of the study, special attention was paid to age-related atrophic degenerative muscle changes. Scientists decided to check whether their technique can restore the muscle strength of elderly mice to the level of young.First, tetanic * strength was established in the muscles of mice aged 4 and 27 months.Tetanus * - a condition of prolonged muscle contraction.
As expected, in older mice this strength was significantly lower than in young mice, which indicates an age factor ( 4h ).Then, MuSC cells were isolated from mice aged 20-24 months, which subsequently underwent a reprogramming process, and then transplanted into cardiotoxin-damaged muscles of mice aged 20 months. After 30 days, tetanic strength measurements were repeated.The muscles into which the cells were transplanted without reprogramming showed the same results as the normal muscles of elderly mice without any intervention. But the muscles where the cells with reprogramming were placed showed tetanic forces comparable to the muscles of young mice ( 4h ).It follows that temporary reprogramming in combination with MuSC-based therapy can restore the physiological function of aging muscles to the functions of young muscles.Carrying out a similar procedure with muscles and human cells showed similar positive results ( 4i and 4j ).For a more detailed familiarization with the nuances of the study, I recommend that you look into the report of scientists and additional materials to it.Epilogue
In the study we examined today, scientists were able to demonstrate a temporary reprogramming of cells that can fight the signs of cell aging and not affect cellular identity.Cells of older people that have undergone reprogramming begin to demonstrate the characteristics of young cells after a few days. In this case, the effect persists for six days after cessation of treatment.This technique is important not only in terms of the general rejuvenation of cells and tissues, but also more specifically in the aspect of combating the effects of age-related diseases, such as osteoarthritis.In the future, scientists intend to improve their methodology. Firstly, as the researchers themselves say, it is necessary to conduct a lot of tests in the laboratory and fully verify the safety of the method at the cell level. After that, it will be possible to begin its use at the tissue level.Is humanity ready for eternal youth in terms of morality, ethics and psychology? The question is complex, but it has a lot of answers. However, the purpose of this study is not in the search for immortality, but in the search for new effective methods of combating various diseases that arise in connection with the inevitable aging of the body. Old age is not as bad as the diseases that come with it. The same can be said about any age, for what joy is there in mythical eternal youth, if for all this eternity a person will struggle with some sore?Friday off-top:
, , , , . ( / Anastatica hierochuntica).
Thank you for your attention, stay curious and have a great weekend everyone, guys! :)A bit of advertising :)
Thank you for staying with us. Do you like our articles? Want to see more interesting materials? Support us by placing an order or recommending to your friends, cloud VPS for developers from $ 4.99 , a unique analog of entry-level servers that was invented by us for you: The whole truth about VPS (KVM) E5-2697 v3 (6 Cores) 10GB DDR4 480GB SSD 1Gbps from $ 19 or how to divide the server? (options are available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).Dell R730xd 2 times cheaper at the Equinix Tier IV data center in Amsterdam? Only we have 2 x Intel TetraDeca-Core Xeon 2x E5-2697v3 2.6GHz 14C 64GB DDR4 4x960GB SSD 1Gbps 100 TV from $ 199 in the Netherlands!Dell R420 - 2x E5-2430 2.2Ghz 6C 128GB DDR3 2x960GB SSD 1Gbps 100TB - from $ 99! Read about How to Build Infrastructure Bldg. class c using Dell R730xd E5-2650 v4 servers costing 9,000 euros for a penny?