 In modern technology, from smartphones to electric vehicles, lithium-ion batteries are used. This type of energy source has several advantages (large capacity, low self-discharge, etc.), but there are also disadvantages. And if the loss of capacity during prolonged exposure to low temperatures is annoying, but not the end of the world, then flammability is a serious matter. The possibility of ignition of the lithium-ion battery is explained by the presence of liquid electrolyte in it, which can be ignited if the battery is damaged or defective. One of the solutions to this problem was the introduction of special flame retardants (substances that provide refractoriness by slowing the combustion process) into the electrolyte. However, a much more interesting solution to this hot problem is to replace liquid electrolyte with solid-state (SSE - Solid-State Electrolyte).The problem is that SSEs, which in theory should provide better refractoriness, are themselves quite combustible, in view of the materials used to reduce their fragility. The problem is there, but scientists from the American Chemical Society (ACS), they said, have found a solution. What materials were used to create the new type of SSE, what properties does the new type of battery have, and what temperatures can it withstand? We learn about this from the report of the research group. Go.What materials were used to create the new type of SSE, what properties does the new type of battery have, and what temperatures can it withstand? We learn about this from the report of the research group. Go.What materials were used to create the new type of SSE, what properties does the new type of battery have, and what temperatures can it withstand? We learn about this from the report of the research group. Go.
In modern technology, from smartphones to electric vehicles, lithium-ion batteries are used. This type of energy source has several advantages (large capacity, low self-discharge, etc.), but there are also disadvantages. And if the loss of capacity during prolonged exposure to low temperatures is annoying, but not the end of the world, then flammability is a serious matter. The possibility of ignition of the lithium-ion battery is explained by the presence of liquid electrolyte in it, which can be ignited if the battery is damaged or defective. One of the solutions to this problem was the introduction of special flame retardants (substances that provide refractoriness by slowing the combustion process) into the electrolyte. However, a much more interesting solution to this hot problem is to replace liquid electrolyte with solid-state (SSE - Solid-State Electrolyte).The problem is that SSEs, which in theory should provide better refractoriness, are themselves quite combustible, in view of the materials used to reduce their fragility. The problem is there, but scientists from the American Chemical Society (ACS), they said, have found a solution. What materials were used to create the new type of SSE, what properties does the new type of battery have, and what temperatures can it withstand? We learn about this from the report of the research group. Go.What materials were used to create the new type of SSE, what properties does the new type of battery have, and what temperatures can it withstand? We learn about this from the report of the research group. Go.What materials were used to create the new type of SSE, what properties does the new type of battery have, and what temperatures can it withstand? We learn about this from the report of the research group. Go.Study basis
As we know, lithium-ion batteries (hereinafter LIA) are present literally everywhere. Such an increased demand for LIA has led manufacturers and scientists to begin to look for new ways to improve their quality in terms of capacity, because everyone wants the battery to last longer, discharge more slowly and charge faster. However, the pursuit of longevity and “power” of batteries has thrown aside safety issues, in particular fire issues. The authors of the study note that lithium dendrites, which gradually increase cycle by cycle at high current densities, can penetrate the separator that separates the battery electrodes and cause a short circuit.There are many solutions to the problem of ignition within the liquid electrolyte: coating the separator with ceramic particles, introducing flame retardants into the electrolyte itself, a built-in temperature regulator, fireproof encapsulation by means of polymers, etc.If we turn from liquid electrolyte to solid-state, then a problem arises with the formation of dendritic * Li due to inhomogeneous deposition of lithium.
Dendrite * - complex crystalline formations resembling a branching tree.
Such lithium “stalagmites” can penetrate the separator and even the cathode, which can lead to a short circuit, overheating, fire, and even explosion. In addition to the danger of such a process, there is also a negative effect on the efficiency of the battery in which lithium dendrites are formed.Currently, existing solid-state electrolytes can be divided into three main categories: inorganic (ceramic / glass), polymer (SPE), and hybrid.Inorganic solid-state electrolytes are interesting in that they have the highest ionic conductivity among all types of SSE. Previous studies described superionic lithium conductors with a conductivity of 25 mS cm cm −1 for Li 9.54 Si 1.74 P 1.44 S11.7 Cl 0.3 , which exceeds the performance of liquid electrolytes.Siemens (cm) - unit of electrical conductivity (1 cm = 1 / ohm); mS (millisiemens) = 10 −3 cm.
However, the instability of air, the fragility of materials, the large interfacial impedance, and the fact that Li in any case penetrates inorganic SSEs after reaching a critical current density, impedes the full use of inorganic SSEs in lithium-ion batteries.If we talk about polymer solid-state electrolytes (SPE), then they mostly consist of solid polymers and lithium salts, where solid impurities serve as lithium-ion conductors. The most studied at the moment is the combination of lithium salts and polyethylene oxide (hereinafter PEO). This structure has a low cost, high lithium-ion conductivity (in comparison with other SPEs) and a fairly low weight, which is important for portable devices. However, the internal softness of this polymer system makes it unable to suppress the spread of lithium dendrites. In other words, there is potential, but it does not solve the necessary problem, which is present in other types of solid-state electrolytes.They tried to solve this problem by reinforcing with nanoparticles, cross-linking and tying a “flexible” electrolyte to a rigid carrier. Despite these complex manipulations, the resulting composite polymer SSEs still remain flammable ( 1a ).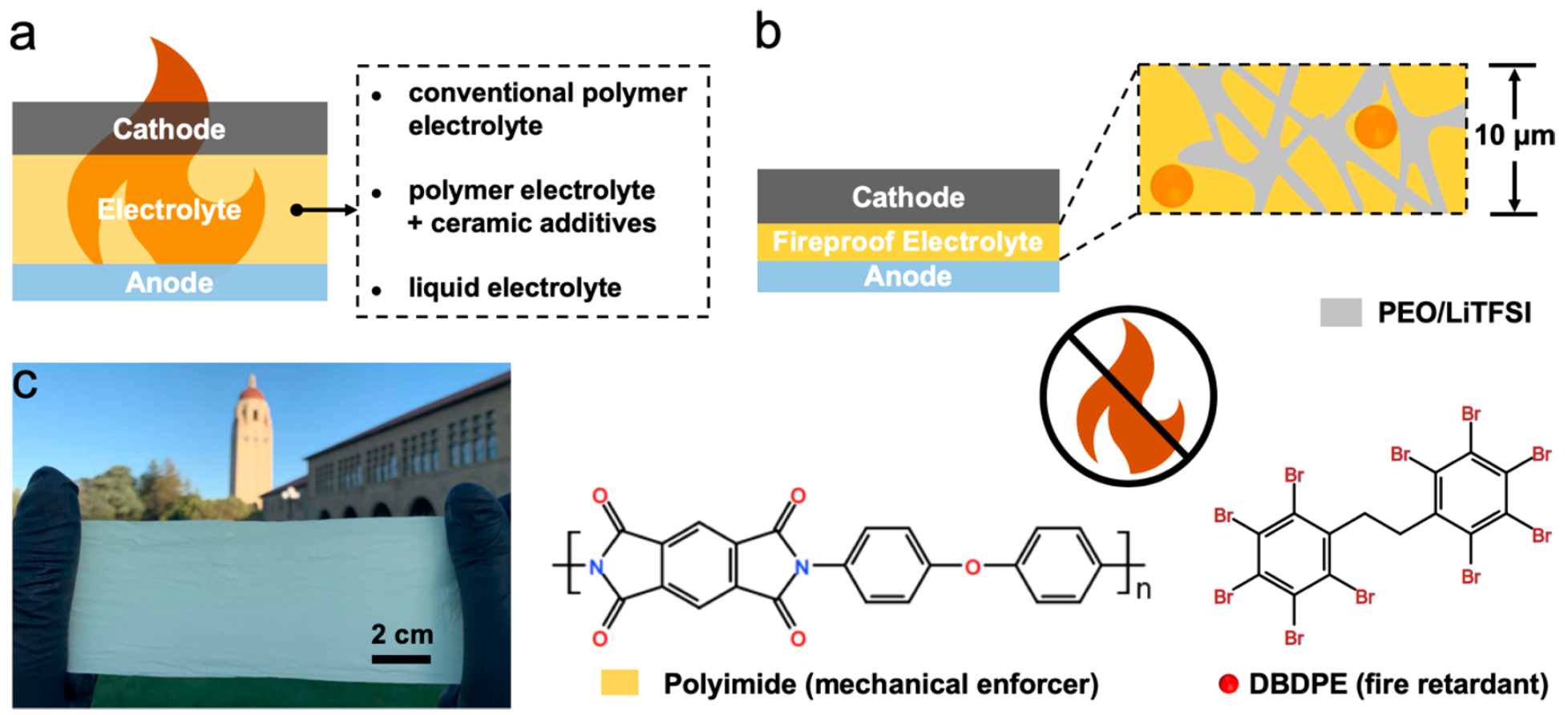 Image No. 1Before conducting the actual study, the scientists tested the flammability of traditional nanocomposite SSEs - PEO / LiTFSI / LLZO and PEO / LiTFSI / Al 2 O 3 , which, as expected, were highly flammable.
Image No. 1Before conducting the actual study, the scientists tested the flammability of traditional nanocomposite SSEs - PEO / LiTFSI / LLZO and PEO / LiTFSI / Al 2 O 3 , which, as expected, were highly flammable.PEO - polyethylene oxide;
LiTFSI - lithium bis (trifluoromethanesulfonyl) imide;
LLZO - Li 7 La 3 Zr 2 O 12 ;
Al 2 O 3 - alumina;
In view of this, the scientists decided to offer their own version of refractory and ultralight SSE with excellent electrochemical characteristics for lithium batteries. The design principles of a flame retardant polymer-polymer solid-state electrolyte are shown in 1b .Research results
The composite SSE was made from porous bifunctional polyimide (PI) and lithium ion conductive SPE fillers. The bifunctional base consists of a durable porous polyimide (PI) film 10 microns thick and lightweight flame-retardant decabromodiphenylethane (DBDPE). The latter is not only very durable, which ensures the prevention of the potential penetration of lithium dendrites, but also is fireproof.Fillers are composed of PEO / LiTFSI, which provides high ionic conductivity SSE.The polymer-polymer nature of the composite electrolyte provides a potentially high energy density for a fully charged battery. That is, this SSE is not only fireproof, but also increases battery capacity.When thermal acceleration occurs in a PI / DBDPE / PEO / LiTFSI solid state electrolyte battery, flame-retardant non-combustible PI DBDPE effectively suppresses the burning of combustible PEO / LiTFSI.At the very beginning, a solution of polyamic acid (PAA) and DBDPE was prepared. The solution was then applied to the glass substrate using a squeegee to obtain a PAA / DBDPE film. To obtain porosity on PAA / DBDPE, a solution of dimethylacetamide and ethanol (DMAC / EtOH) was used. Upon completion of drying, the PAA / DBDPE film was imidized (cyclization of amido acid to polyimide) at 300 ° C to obtain the final porous PI / DBDPE film.After drying, the PAA / DBDPE porous film was imidized at 300 ° C to obtain the final PI / DBDPE porous film (photo film for 1 s ).Using scanning electron microscopy, detailed film characteristics were obtained. Figure 2a shows the morphology of the film on the front side (external, i.e., in contact with air) during the smoothing process with a squeegee.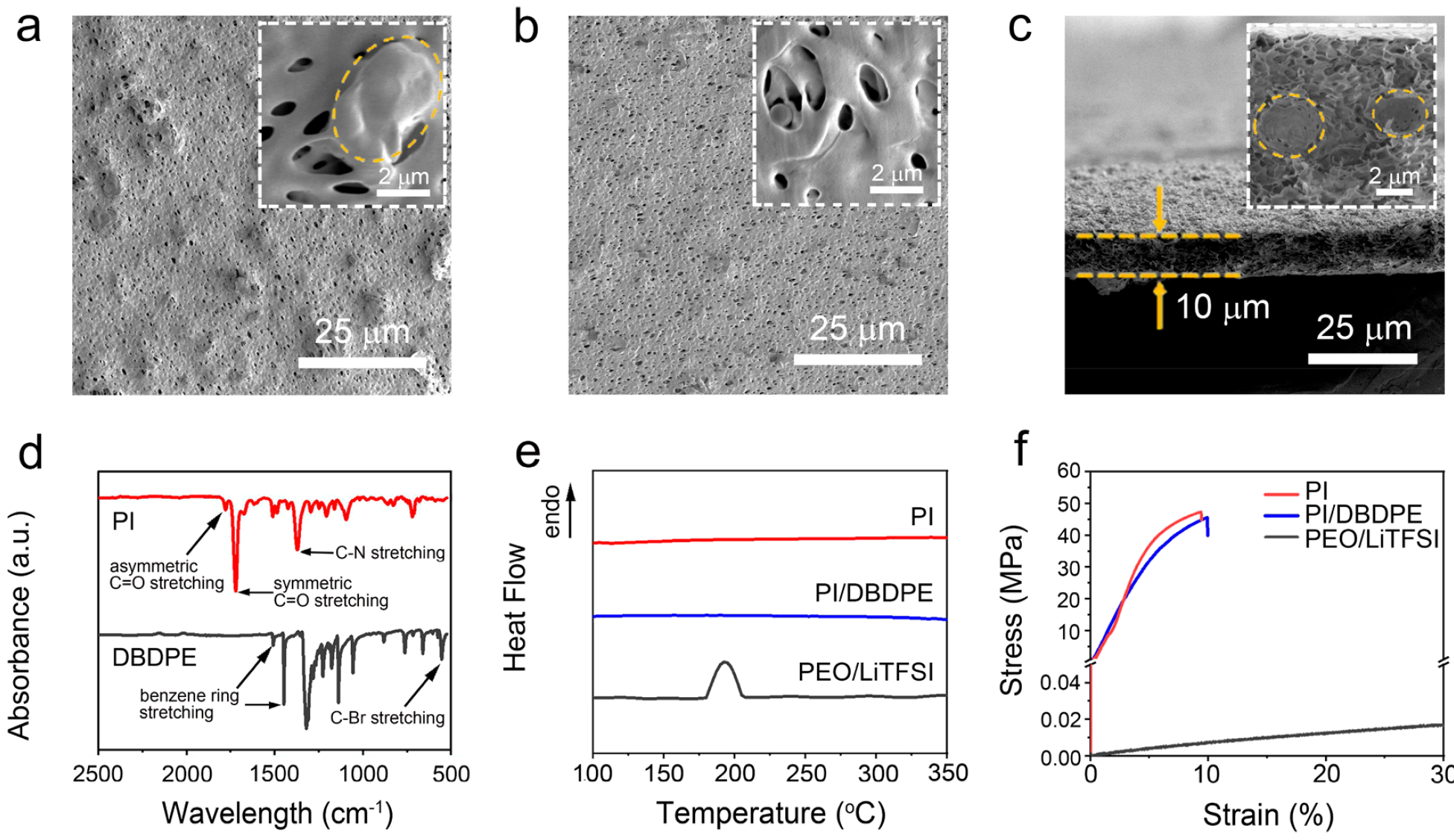 Image No. 2As we see, the pores and particles of DBDPE were evenly distributed on the surface of the outer side of the PI / DBDPE film. According to microscopy, the pore diameter was about 500 nm. On 2a orange dotted line marked particles DBDPE, the dimensions of which ranged from submicron to several microns.The morphology of the back (facing the glass) side of the film is shown in 2b, where it is seen that there are fewer DBDPE particles than on the front side of the film. The pore sizes on this side are the same as on the front, i.e. 500 nm.The cross-sectional photograph of the PI / DBDPE film shows excellent uniformity with a constant thickness of about 10 μm ( 2c ). It was found that the use of a squeegee allows you to adjust the film thickness from 10 to 25 microns. 2c also shows DBDPE particles (orange dotted line), which confirms the good porosity of the back side of the film. Inside the film, the pores are also well distributed, and their diameter is 500 nm, as in other parts of the film.On image 2dFourier transform infrared spectroscopy of PI film and DBDPE particles is shown. All peaks in the spectrum correspond well to typical PI and DBDPE, which confirms the chemical composition of the synthesized PI, DBDPE, and PI / DBDPE films.Given the functions of the separator, heat resistance is an extremely important parameter for this part of the battery. The low melting point of the separator can cause severe shrinkage of the separator at an early stage of internal short circuit, which can accelerate the process of thermal acceleration. Figure 2e shows the results of DSC (differential scanning calorimetry - thermal analysis method) PI / DBDPE, PI, and PEO / LiTFSI films.No endothermic was detected for PI / DBDPE and PI films *peaks in the entire scan range. But in the case of the PEO / LiTFSI film, there were peaks at ~ 180 ° C. Therefore, PI / DBDPE and PI films showed much higher thermal stability than PEO / LiTFSI films.
Image No. 2As we see, the pores and particles of DBDPE were evenly distributed on the surface of the outer side of the PI / DBDPE film. According to microscopy, the pore diameter was about 500 nm. On 2a orange dotted line marked particles DBDPE, the dimensions of which ranged from submicron to several microns.The morphology of the back (facing the glass) side of the film is shown in 2b, where it is seen that there are fewer DBDPE particles than on the front side of the film. The pore sizes on this side are the same as on the front, i.e. 500 nm.The cross-sectional photograph of the PI / DBDPE film shows excellent uniformity with a constant thickness of about 10 μm ( 2c ). It was found that the use of a squeegee allows you to adjust the film thickness from 10 to 25 microns. 2c also shows DBDPE particles (orange dotted line), which confirms the good porosity of the back side of the film. Inside the film, the pores are also well distributed, and their diameter is 500 nm, as in other parts of the film.On image 2dFourier transform infrared spectroscopy of PI film and DBDPE particles is shown. All peaks in the spectrum correspond well to typical PI and DBDPE, which confirms the chemical composition of the synthesized PI, DBDPE, and PI / DBDPE films.Given the functions of the separator, heat resistance is an extremely important parameter for this part of the battery. The low melting point of the separator can cause severe shrinkage of the separator at an early stage of internal short circuit, which can accelerate the process of thermal acceleration. Figure 2e shows the results of DSC (differential scanning calorimetry - thermal analysis method) PI / DBDPE, PI, and PEO / LiTFSI films.No endothermic was detected for PI / DBDPE and PI films *peaks in the entire scan range. But in the case of the PEO / LiTFSI film, there were peaks at ~ 180 ° C. Therefore, PI / DBDPE and PI films showed much higher thermal stability than PEO / LiTFSI films.Endothermic reactions * - a chemical reaction in which heat is absorbed.
Figure 2f is a film strain diagram obtained from tensile tests. The porous PI / DBDPE film showed a Young's modulus of 440 MPa, which was slightly lower than that of a pure porous PI film (470 MPa), but almost 4 orders of magnitude higher than that of PEO / LiTFSI (0.1 MPa). Consequently, the PEO / LiTFSI film in this test also loses much to the other two, since its mechanical strength is quite small.For a quantitative analysis of DBDPE refractoriness, self-extinguishing time (SET) self-extinguishing time of PEO / LiTFSI electrolytes with different DBDPE concentrations was measured ( 3a) SET was obtained by normalizing the flame burning time with respect to the mass of the electrolyte. The initial PEO / LiTFSI was flammable with a SET value of about 120 s / g.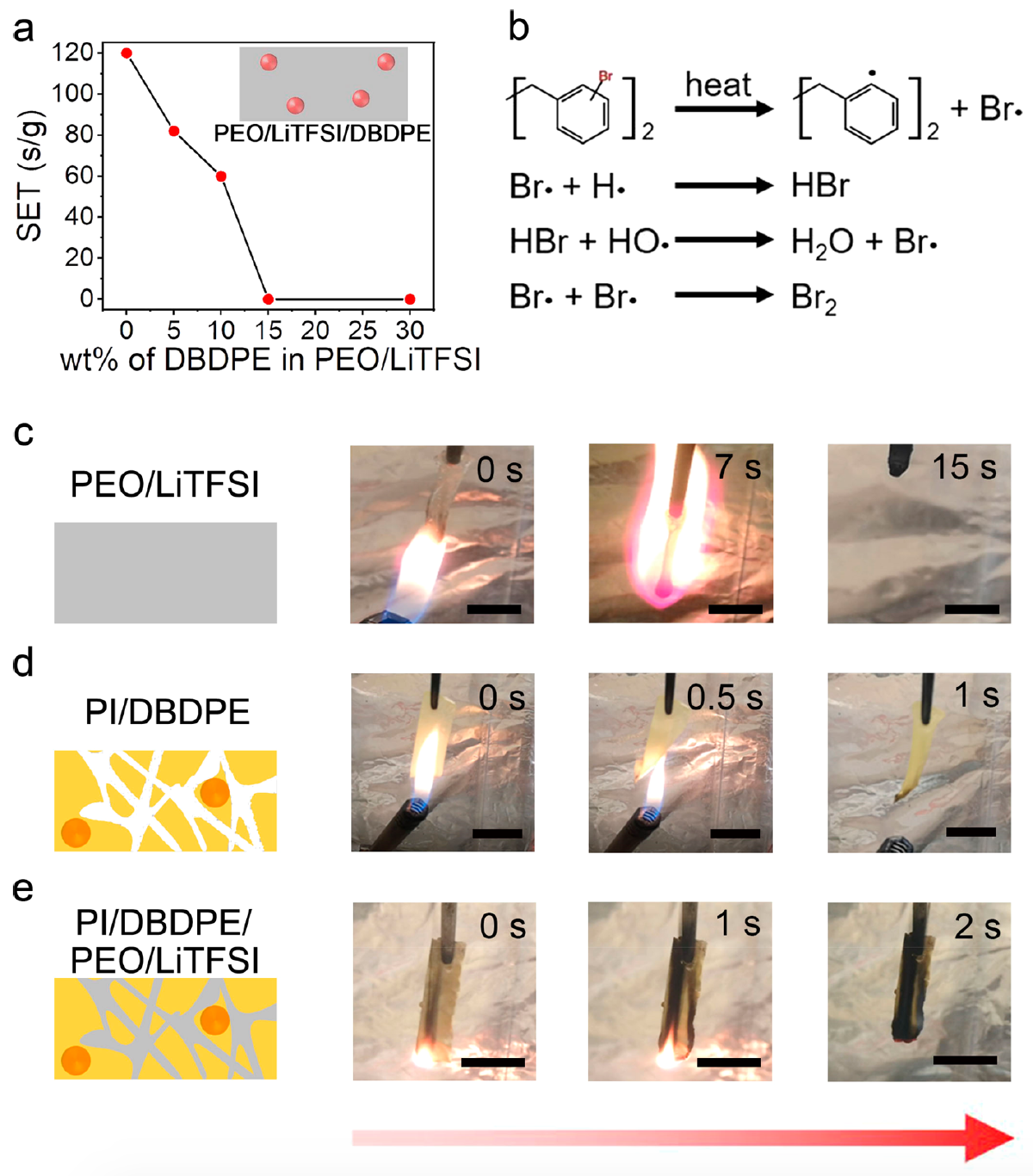 Image # 3SET PEO / LiTFSI gradually decreased with the addition of DBDPE. This suggests that the flammability of PEO / LiTFSI decreased with increasing percentage of DBDPE. The SET value dropped to zero when the DBDPE concentration reached 15%.Scientists have suggested that the DBDPE fire resistance mechanism is based on the free radical uptake reaction ( 3b ), since DBDPE can decompose to form bromine free radicals (Br •) when heated. Highly reactive H • and OH • radicals released by a burning electrolyte can be captured by Br •, weakening or terminating combustion reactions.Moreover, gas-phase products, such as HBr, H 2 O and Br 2 , released in the free radical absorption reaction, limit thermal and mass transfer. These gaseous products dilute the oxygen concentration between the heat source and the electrolyte, thereby slowing down the combustion process.The effectiveness of DBDPE in suppressing combustion has been tested in practice by tests with actual flame. It was determined that the percentage of DBDPE in the PI film is 30%. PEO / LiTFSI and PI / DBDPE films served as control samples. PI / DBDPE / PEO / LiTFSI film showed a difference in the results of fire tests.As seen in image 3c, PEO / LiTFSI without DBDPE instantly ignited as soon as the flame approached the film, and then quickly burned out.A 3d image shows a test of a PI / DBDPE film that began to curl when exposed to heat but did not light up.Filling the pores of the PI / DBDPE film with combustible PEO / LiTFSI led to the fact that the ignition and combustion of PEO / LiTFSI was effectively suppressed, and the SSE remained intact due to the DBDPE refractory material in it ( 3e ).Next, PI and PI / PEO / LiTFSI films were compared during flammability tests. The pure PI film was completely refractory. But PI / PEO / LiTFSI caught fire very quickly, which indicates the importance of DBDPE in suppressing the ignition of solid-state electrolytes.Following the ignition test, scientists conducted a cyclic test to assess the mechanical stability of SSE PI / DBDPE / PEO / LiTFSI during lithiation (lithium deposition) and during desorption (in this case, lithium removal) ( 4a ).
Image # 3SET PEO / LiTFSI gradually decreased with the addition of DBDPE. This suggests that the flammability of PEO / LiTFSI decreased with increasing percentage of DBDPE. The SET value dropped to zero when the DBDPE concentration reached 15%.Scientists have suggested that the DBDPE fire resistance mechanism is based on the free radical uptake reaction ( 3b ), since DBDPE can decompose to form bromine free radicals (Br •) when heated. Highly reactive H • and OH • radicals released by a burning electrolyte can be captured by Br •, weakening or terminating combustion reactions.Moreover, gas-phase products, such as HBr, H 2 O and Br 2 , released in the free radical absorption reaction, limit thermal and mass transfer. These gaseous products dilute the oxygen concentration between the heat source and the electrolyte, thereby slowing down the combustion process.The effectiveness of DBDPE in suppressing combustion has been tested in practice by tests with actual flame. It was determined that the percentage of DBDPE in the PI film is 30%. PEO / LiTFSI and PI / DBDPE films served as control samples. PI / DBDPE / PEO / LiTFSI film showed a difference in the results of fire tests.As seen in image 3c, PEO / LiTFSI without DBDPE instantly ignited as soon as the flame approached the film, and then quickly burned out.A 3d image shows a test of a PI / DBDPE film that began to curl when exposed to heat but did not light up.Filling the pores of the PI / DBDPE film with combustible PEO / LiTFSI led to the fact that the ignition and combustion of PEO / LiTFSI was effectively suppressed, and the SSE remained intact due to the DBDPE refractory material in it ( 3e ).Next, PI and PI / PEO / LiTFSI films were compared during flammability tests. The pure PI film was completely refractory. But PI / PEO / LiTFSI caught fire very quickly, which indicates the importance of DBDPE in suppressing the ignition of solid-state electrolytes.Following the ignition test, scientists conducted a cyclic test to assess the mechanical stability of SSE PI / DBDPE / PEO / LiTFSI during lithiation (lithium deposition) and during desorption (in this case, lithium removal) ( 4a ). Image No. 4The current density was first set to 0.05 mA cm -2 at 60 ° C to activate symmetrical Li / SSE / Li cells. After the current density was increased to 0.1 in the sixth cycle, a short circuit occurred immediately in pure PEO / LiTFSI ( 4b ). Moreover, PI / DBDPE / PEO / LiTFSI showed much more stable characteristics for 300 hours at 60 ° C. This suggests that such structures perfectly prevent the formation of lithium dendrites.Next, PI / DBDPE / PEO / LiTFSI electrochemical tests were performed at 60 ° C. The cathode of the test battery was made of LiFePO 4 (LFP), and the anode of lithium. The control group of batteries was made on the same principle, but without turning on PEO / LiTFSI.As seen in 4c , the PI / DBDPE / PEO / LiTFSI batteries showed excellent performance. Voltage profiles at different speeds showed a clean plateau of about 3.45 V, which is typical for LFP cathodes. The specific capacity of LFP / PI / DBDPE / PEO / LiTFSI / Li was quite high for all cycle options ( 4d ): 163 mAh g -1 , 152 mAh g -1 , 143 mAh g -1 and 131 mAh g -1 . But for LFP / PEO / LiTFSI / Li this indicator was lower: 134 mAh g-1 , 129 mAh g -1 , 122 mAh g -1 and 115 mAh g -1 ( 4e ). The combination of these data indicates the high performance of PI / DBDPE / PEO / LiTFSI.Next, we compared the thermal stability of PI / DBDPE with a PE separator and PEO / LiTFSI ( 5a ).
Image No. 4The current density was first set to 0.05 mA cm -2 at 60 ° C to activate symmetrical Li / SSE / Li cells. After the current density was increased to 0.1 in the sixth cycle, a short circuit occurred immediately in pure PEO / LiTFSI ( 4b ). Moreover, PI / DBDPE / PEO / LiTFSI showed much more stable characteristics for 300 hours at 60 ° C. This suggests that such structures perfectly prevent the formation of lithium dendrites.Next, PI / DBDPE / PEO / LiTFSI electrochemical tests were performed at 60 ° C. The cathode of the test battery was made of LiFePO 4 (LFP), and the anode of lithium. The control group of batteries was made on the same principle, but without turning on PEO / LiTFSI.As seen in 4c , the PI / DBDPE / PEO / LiTFSI batteries showed excellent performance. Voltage profiles at different speeds showed a clean plateau of about 3.45 V, which is typical for LFP cathodes. The specific capacity of LFP / PI / DBDPE / PEO / LiTFSI / Li was quite high for all cycle options ( 4d ): 163 mAh g -1 , 152 mAh g -1 , 143 mAh g -1 and 131 mAh g -1 . But for LFP / PEO / LiTFSI / Li this indicator was lower: 134 mAh g-1 , 129 mAh g -1 , 122 mAh g -1 and 115 mAh g -1 ( 4e ). The combination of these data indicates the high performance of PI / DBDPE / PEO / LiTFSI.Next, we compared the thermal stability of PI / DBDPE with a PE separator and PEO / LiTFSI ( 5a ).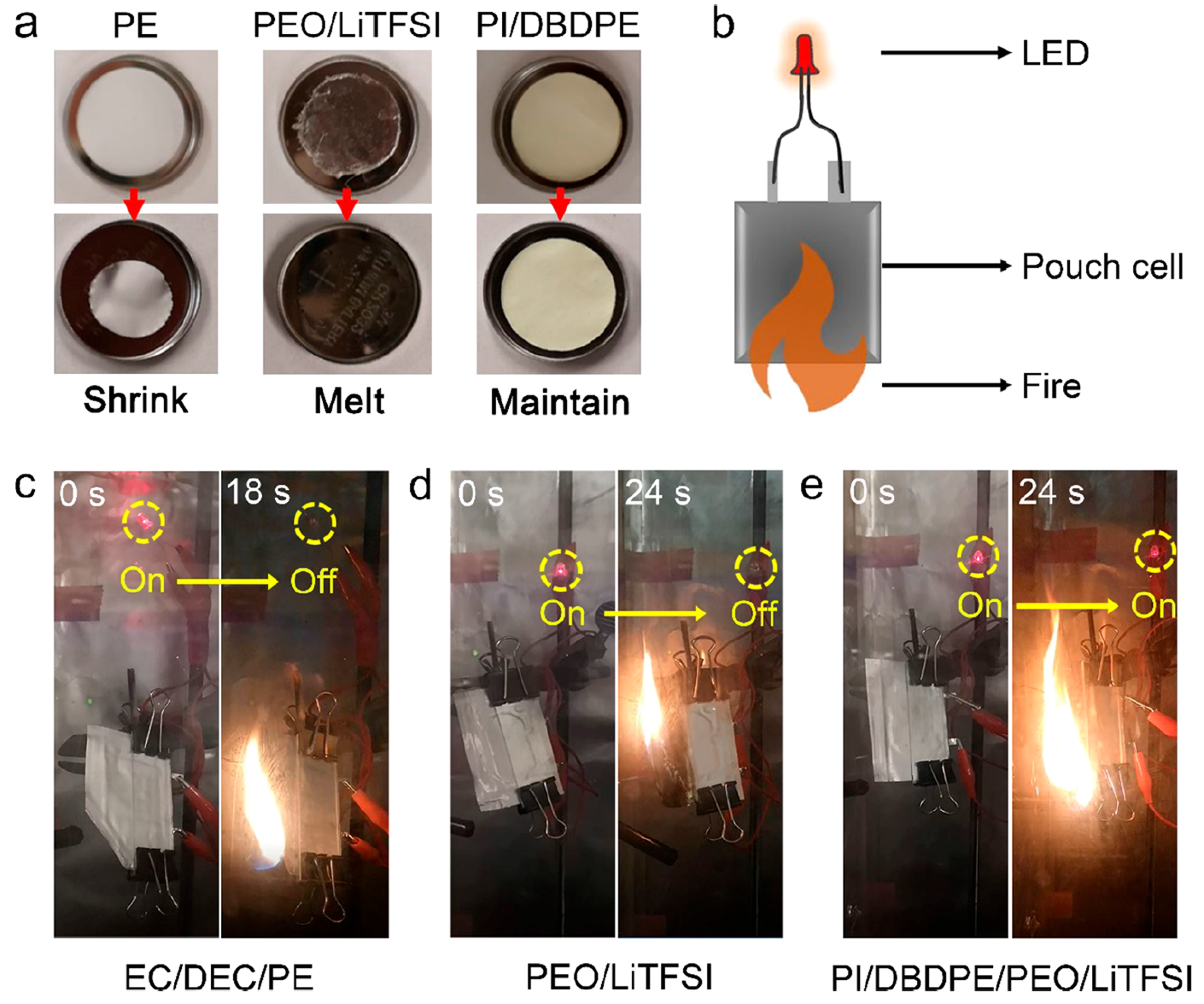 Image No. 5When exposed to a temperature of 150 ° C for 30 minutes, the area of the separator was halved, while PEO / LiTFSI melted. In contrast, in PI / DBDPE, no significant changes in film size and morphology were observed.For greater effect, scientists decided to conduct another test - a test of thermal abuse (at 5bshows the battery operation during this test).The cathode was LFP, and the anode was Li 4 Ti 5 O 12 (LTO). The only difference between the tested samples was electrolyte.The flame affected three sample variants ( 5c - 5e ): liquid electrolyte / polymer (EC / DEC / PE - ethylene carbonate / diethyl carbonate / polyethylene); conventional polymer electrolyte and test SSE (PI / DBDPE / PEO / LiTFSI).Samples EC / DEC / PE and PEO / LiTFSI failed to light up the LEDs after burning for 18 and 24 seconds, respectively. Sample PI / DBDPE / PEO / LiTFSI continued to support LED operation even after 24 seconds of flame exposure.This test is a hyperbolized option for misuse of batteries. However, even he showed that PI / DBDPE / PEO / LiTFSI has excellent heat resistance.
Image No. 5When exposed to a temperature of 150 ° C for 30 minutes, the area of the separator was halved, while PEO / LiTFSI melted. In contrast, in PI / DBDPE, no significant changes in film size and morphology were observed.For greater effect, scientists decided to conduct another test - a test of thermal abuse (at 5bshows the battery operation during this test).The cathode was LFP, and the anode was Li 4 Ti 5 O 12 (LTO). The only difference between the tested samples was electrolyte.The flame affected three sample variants ( 5c - 5e ): liquid electrolyte / polymer (EC / DEC / PE - ethylene carbonate / diethyl carbonate / polyethylene); conventional polymer electrolyte and test SSE (PI / DBDPE / PEO / LiTFSI).Samples EC / DEC / PE and PEO / LiTFSI failed to light up the LEDs after burning for 18 and 24 seconds, respectively. Sample PI / DBDPE / PEO / LiTFSI continued to support LED operation even after 24 seconds of flame exposure.This test is a hyperbolized option for misuse of batteries. However, even he showed that PI / DBDPE / PEO / LiTFSI has excellent heat resistance.Video materials for the study:
№1: PEO/LiTFSI/LLZO.
№2: PEO/LiTFSI/Al2O3.
№3: PEO/LiTFSI/DBDPE ( DBDPE 15 %).
№4: PEO/LiTFSI.
№5: PI/DBDPE.
№6: PI/DBDPE/PEO/LiTFSI.
№7: PI.
№8: PI/PEO/LiTFSI.
№9: EC/DEC/PE.
№10: PEO/LiTFSI.
№11: PI/DBDPE/PEO/LiTFSI.
For a more detailed familiarization with the nuances of the study, I recommend that you look into the report of scientists and additional materials to it.Epilogue
In this work, scientists have demonstrated that the creation of safer batteries, without compromising their capacitive characteristics, is quite possible. For this, we used a porous PI film with the flame retardant material DBDPE as the base and PEO / LiTFSI as the ion-conducting filler. The main achievement of the new hybrid battery is its fire resistance. However, this is not the only one in which this invention surpasses its predecessors. So, for example, a hybrid battery has demonstrated excellent cyclic stability and impressive capacity.In the pursuit of increasing one indicator, others often suffer. So it was with batteries, when all the attention was paid to the capacity and duration of their life, and the problem of ignition remained on the sidelines. Of course, now many scientists are developing new types of batteries that are different from lithium-ion, which could combine all the possible advantages of their predecessors, leaving behind their drawbacks. However, while there are no such super-batteries, you should not disdain to improve what is available.Thank you for your attention, stay curious and have a good working week, guys. :)A bit of advertising :)
Thank you for staying with us. Do you like our articles? Want to see more interesting materials? Support us by placing an order or recommending to your friends, cloud VPS for developers from $ 4.99 , a unique analog of entry-level servers that was invented by us for you: The whole truth about VPS (KVM) E5-2697 v3 (6 Cores) 10GB DDR4 480GB SSD 1Gbps from $ 19 or how to divide the server? (options are available with RAID1 and RAID10, up to 24 cores and up to 40GB DDR4).Dell R730xd 2 times cheaper at the Equinix Tier IV data center in Amsterdam? Only we have 2 x Intel TetraDeca-Core Xeon 2x E5-2697v3 2.6GHz 14C 64GB DDR4 4x960GB SSD 1Gbps 100 TV from $ 199 in the Netherlands!Dell R420 - 2x E5-2430 2.2Ghz 6C 128GB DDR3 2x960GB SSD 1Gbps 100TB - from $ 99! Read about How to Build Infrastructure Bldg. class c using Dell R730xd E5-2650 v4 servers costing 9,000 euros per penny?